Abstract
Background
Although combined oral contraceptives (COC) are considered a transient risk factor for venous thromboembolism (VTE), the risk of recurrence after discontinuation of anticoagulation is unclear. Few studies have focused on the risk of recurrence in this group; studies report variable results and are limited by small sample size. The risk of recurrence appears to be low, but this could relate to the young age of affected women. Deciphering the absolute VTE recurrence risk after a COC-associated VTE is crucial in helping clinicians and patients decide if anticoagulation could be discontinued after the initial treatment period.
Objectives
The objectives of this systematic review and meta-analysis are to estimate the incidence of recurrent VTE among women with COC-associated VTE, compared with women with unprovoked VTE.
Methods
We searched the following databases: Cochrane Central Register of Controlled Trial, Cochrane Database of Systematic Reviews, Embase Classic +Embase, and Medline ALL, all from the OvidSP platform, from the database's inception to July 2020. Additional studies were identified by screening citations from included studies.
Prospective cohort studies, randomized controlled trials (RCTs), and meta-analyses of prospective cohort studies or RCTs were reviewed by two authors for study inclusion (Figure 1). Studies were included if women had objectively confirmed COC-associated VTE, received a minimum of three months of anticoagulation, discontinued COC prior to or at time of discontinuation of anticoagulation, time of follow-up began after anticoagulation was stopped, and recurrent VTE data was available. Studies were excluded if patients were systematically treated with an alternative pharmacologic agent intended to reduce the risk of recurrent VTE such as aspirin. Authors of identified papers were contacted for additional data on critical variables. If there were multiple publications from a cohort, the study with the longest follow up was included.
Two authors extracted study data and assessed included studies for risk of bias using the Newcastle Ottawa Scale. Meta-analysis was done using a random effects Poisson regression model. Heterogeneity was assessed using the I-squared measure.
Results
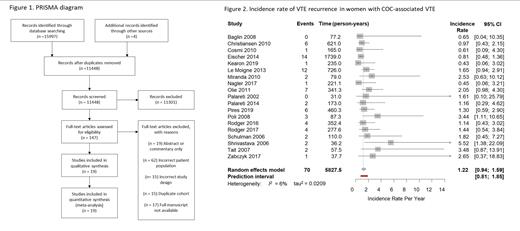
Our systematic review included 19 studies with a total of 1,537 women (5,828 patient years of follow up) with an index COC-associated VTE, and 1,974 women (7,798 patient years of follow up) with an index unprovoked VTE. Authors contributed additional unpublished data in 16 of the 19 studies. Overall, studies were at low risk of bias, with a mean of 7 stars (out of a possible 9) in the Newcastle Ottawa Scale.
Among the 19 studies, the incidence rate of VTE recurrence in women with COC-associated VTE was 1.22 per patient year (95% confidence interval (CI) 0.94 to 1.59, I 2 = 6.4%, 95% prediction interval (PI) 0.81 to 1.85) (Figure 2). The incidence rate of VTE recurrence in women with an index unprovoked VTE not associated with COC was 3.89 per patient year (95% CI 2.98 to 5.07, I 2 =74.2%, 95% PI 1.37 to 11.03).
The unadjusted incidence rate ratio of recurrent VTE comparing women with COC-associated events to women with unprovoked events was 0.34 (95% CI 0.26 to 0.45, I 2 = 2.6%, 95% PI 0.26 to 0.46). Only three studies had age-adjusted comparisons, but each with a different effect measure so they could not be combined, with a relative risk ratio 0.4 (95% CI 0.2 to 0.8) (also adjusted for site of VTE and congenital thrombophilia) (Eischer 2014), a hazard ratio of 0.11 (95% CI 0.01 to 0.85) (Kearon 2019), and an incidence rate ratio of 1 (95% CI 0.3 to 3.2) (Le Moigne 2013).
Conclusions
The estimated risk of VTE recurrence after a COC-associated VTE is low, and is lower compared to women with unprovoked VTE, however this comparison may be confounded by age. With only a minority of studies providing age adjusted analyses, the true difference remains unknown. Our meta-analysis is strengthened by the substantial contribution of unpublished data from individual study authors. This can help to guide clinicians and patient shared decision-making on the duration of anticoagulation.
Le Gal: LEO Pharma: Honoraria; BMS: Honoraria; Pfizer: Honoraria; Bayer: Honoraria; Aspen: Honoraria; Sanofi: Honoraria. Schulman: Boehringer-Ingelheim: Research Funding; Octapharma: Research Funding. Skeith: CSL Behring: Research Funding; Leo Pharma: Honoraria; Sanofi: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal